
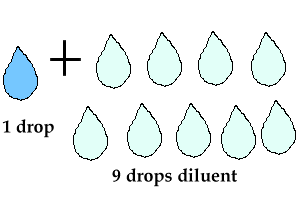
#Easy way to do dilutions serial#
Serial dilutions are often performed in steps of 10 or 100. The initial concentration and target range needed determines the size and number of dilution steps required. Doing this several times results in a range of concentrations.

The diluted sample is then used as the base solution to make an additional dilution. The concentration (or titer) of the last dilution should be 1/8 or simply “8” but not 1:8.To perform a serial dilution, a small amount of a well-mixed solution is transferred into a new container, and additional water or other solvent * is added to dilute the original solution. So you would start with 1 part serum +1 part saline to produce a 1/2 solution, diluted 1/2 to give 1/4, then again to produce 1/8 etc.
#Easy way to do dilutions series#
I think it is simpler to use the convention most of the rest of the world uses where a fraction indicates “in” and a ratio sign (:) indicates “to.” In MLS a doubling dilution of serum would indicate a series of dilution with the final dilution expressed as the concentration of serum in that tube. On the other hand, a 1:2 means 1 part of A added to 2 parts of B for a total dilution of 1 in 3 (a total volume of 3). Therefore I was taught that a 1/2 dilution is 1 part of A to 1 part of B making a total volume of 2. To me “in” means a total volume of solution whereas “to” means a ratio of a certain number of parts of something to a certain number of parts of something else. I think the issue is using the proportional sign (: or “to”) interchangeably with “in.” In Canada where I trained in MLS, 1:2 meant what it looks like as a ratio 1 part to 2 parts for a total dilution of 1 in 3. I think the confusion is more an Americanism than anything else. Patti Jones PhD, DABCC, FACB, is the Clinical Director of the Chemistry and Metabolic Disease Laboratories at Children’s Medical Center in Dallas, TX and a Professor of Pathology at University of Texas Southwestern Medical Center in Dallas. You might want to just check your own MLS and see how they do their dilutions. Either convention works fine as long as it is clear to everyone in the lab what dilution they are actually performing and being asked to perform. However, perhaps the majority of medical laboratory scientists are taught the ratio.
#Easy way to do dilutions plus#
Are they doing a true 1/10 (1 mL sample plus 9 mL diluent) or are they actually doing a 1 to 11 dilution (1 mL sample plus 10 mL diluent)? Your patient results may be different depending on who does the dilution!Ĭoming into this field from a scientific background rather than an MLS background, I prefer the convention of writing a dilution as 1 over something, ½, 1/10, rather than as a ratio, 1:2, 1:10. It’s very important to know how the technologists in the lab are performing that 1 to 10 dilution. I’ve seen 1 to 10 dilutions written both as 1/10 and 1:10. Unfortunately, this problem is prevalent in the laboratory. That will be 1 mL added to 2 mL, for a total of 3 mL, or a 1/3 dilution. In the scientific literature, if you see “1:2”, it means to add 1part to 2 parts.

One is a dilution and the other is a ratio. These two forms are actually not equal, despite the fact that they are used interchangeably in the laboratory. But many times it will be written as 1:2. That’s because I’ve found that the convention for writing dilutions is taught differently at different Medical Laboratory Science (MLS) schools.Ī 1 to 2 dilution should be written as ½. But if you ask people to do a 1 to 2 dilution, you may be surprised to get different results. If you ask someone to dilute a sample in half, pretty much everyone will do it the same way – add an equal volume of sample to an equal volume of diluent, whether that’s 1 mL to 1 mL or 100 µL to 100 µL.


 0 kommentar(er)
0 kommentar(er)
